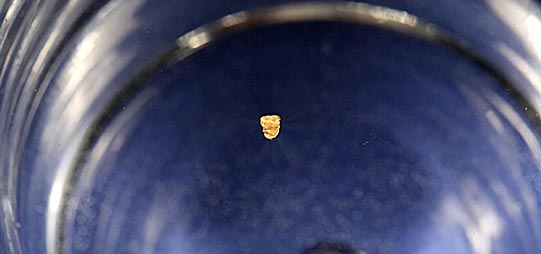


Even though
this piece of gold is 19 times denser than water, it floats with ease.
Floating gold is a serious problem for all gold prospectors because it can wash away faster than anyone can react to save it. This brief page will explain what surface tension is, how it enables gold to float and what to do to prevent it.
In the
following image, the oblong yellow line represents a flake of gold
and the red and cyan spheres below it are grossly out of scale water molecules:
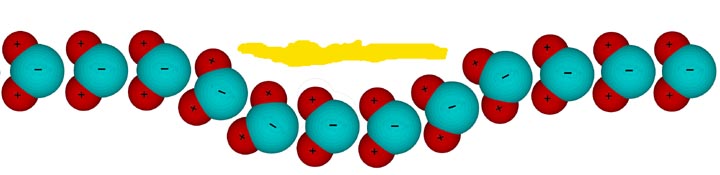
Water consists of two hydrogen atoms (red spheres) attached to a single oxygen atom (cyan spheres.) Although the molecules are electrically neutral, intramolecular forces cause the electrons to spend more time near the oxygen atom than the hydrogen atoms. This give the molecule a polar electric field. They act like magnets except the fields are electric in nature rather than magnetic. Because opposite fields attract, the oxygen atoms are attracted to the hydrogen atoms of any neighboring molecules. This causes them to line up and resist any effort to pull them apart. (While the representation above isn't totally accurate, it's close enough to serve the purposes of this page.) This mutual attract is called surface tension because it manifests itself on the surface of the liquid. It's this force that causes water drops on a waxed car to bead up and raindrops to pull themselves into spheres.
Gold is hydrophobic: it repels water. Because of this, even if the piece of gold is first completely submerged, if it gets near the surface it will throw off the water above it and float. The reason is that the water's surface tension acts like the straps on a trampoline. Climb on one and they will stretch downward but hold you up. In the same way, the weight of the gold will stretch the water links creating the surface tension, but as long as the gold is lighter than the upward force from the surface tension the gold will float.
At room temperature, the strength of pure water's surface tension is 0.0004 pounds per inch. This makes it easy to calculate whether a given piece of gold will float or not. Calculate its circumference in inches, multiply this by the value for the strength of the surface tension and if this number is greater than the weight of the gold, it will float. Since most placer gold is flat and thin, its weight is small relative to its circumference so it will usually float.
All this may
be interesting, but what prospectors really want to know is:
"What is the least amount of surfactant needed to prevent gold
from floating?" A surfactant is any liquid which reduces the
strength of the water's surface tension. They work by wedging
themselves between water molecules, thereby weakening the forces of
attraction between them. It's like the old saying: A chain is only
as strong as its weakest link. In this case the surfactant is
creating billions of weak links in the water's surface tension.
The most common surfactant is Jet Dry. This is a dishwasher additive that prevents water droplets from spotting glasses as they dry in dishwashers. It's preferred by prospectors because it not only does a good job, is easy to find and cheap, but also because unlike soap, it doesn't foam up very much... which is why we want to use as little as possible.
Bubbles and foam can support fine gold and in so doing help it to wash away. The mechanism is different than surface tension but just as problematic. Use too much Jet Dry and you get foam. Use too little and the surface tension isn't weakened enough and gold will float away. To determine the minimum amount of Jet Dry required to prevent floating but minimize foaming, I slowly added Jet Dry a single drop at a time to two cups of water and tested each mixture after thorough stirring to determine at what point gold first stopped floating.
What I found was one drop in two cups had little effect. Two drops prevented the gold from floating, but it often took a second or two before it fell, more than enough time for it to wash away on. say, a Miller table. Three drops in two cups of water turned out to be optimum. Gold instantly fell to the bottom of the glass no matter how carefully it was placed on the water's surface. This works out to 1/8-teaspoon per gallon of water. More Jet Dry can be used if the recovery system being used doesn't cause bubbling and frothing. I have a Miller table set up so that there is absolutely no bubbling and can easily use ten times as much Jet Dry without foaming problems. Panners will want to use the minimum because the panning process invariably agitates the water enough to create bubbles.
Thank you for visiting this page. I hope you found it interesting and helpful. If you'd like to watch a video version of it or any of my other 26 videos about backyard gold prospecting, please click on the following link:
Return to my main page to browse 60 other subjects